
Chemical purity is essential for developing safe medicines and research. Scientists use identification analysis for substances to ensure purity. Discover why HPLC is ideal for chemical purity testing with this comprehensive guide.
(more…)
Carbon-14 radiochemistry refers to radiocarbon dating. It’s a scientific method that determines the age of organic samples. Explore the brief history of carbon-14 radiochemistry to understand its origins and impact on society.
(more…)
Hazardous chemicals have disastrous effects on the environment and humans. Scientists need to safely manage substances to protect everyone around them. Explore why proper chemical storage is important in laboratories to understand their benefits.
(more…)
Chemical synthesis is a unique science that enables drug discovery and other areas of chemical biology. Scientists use compound synthesis and radioactive isotopes to advance research. Keep reading to learn why tritium is so important in compound synthesis for insightful information.
(more…)
Current Good Manufacturing Practice (CGMP) is a set of regulations for the pharmaceutical industry. The strict standards ensure safe drugs for consumers. For more information, read the CGMP guidelines and regulations everyone should know.
(more…)
Technology is a game-changer for several industries, including pharmaceuticals. Companies use the latest technology to improve healthcare, produce drugs, and automate processes. Discover how tech advancements are improving drug development, with specific examples below.
(more…)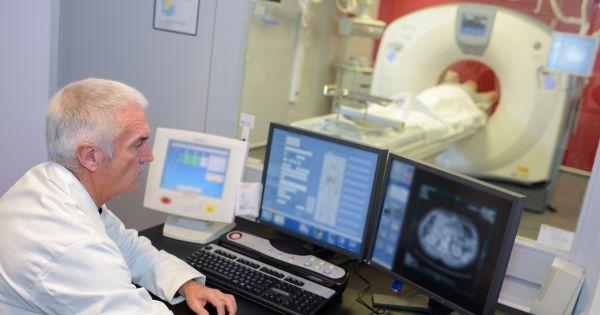
Nuclear medicine is a special area of radiology that utilizes radioactive materials like radiopharmaceuticals to examine organ function. The branch of radiology is highly effective in the medical community. As you explore the benefits of nuclear medicine, you’ll discover its advantages.
(more…)
Radioactive isotopes are suitable for various applications in many fields, such as the medical, agricultural, and food industries. However, their intended purpose depends on specific needs. Learn how radioisotopes can affect your research to ensure your findings’ accuracy.
Read more
When separating chemical compounds, scientists use purification techniques to remove impurities. Given the different properties of compounds, there isn’t a singular method for purification. In fact, purification differs between chemical research fields. Keep reading for insightful information on purification and its variations.
(more…)
Drug manufacturers enhance their credibility with relevant products. To improve or create medications, manufacturers benefit from custom synthesis to streamline production. Understand the advantages of this initiative by learning how custom synthesis differs from standard practices.

Demonstrate your commitment to product safety with a Good Manufacturing Practices (GMP) certification. Become an industry leader with trusted products and satisfied consumers. Discover how long your GMP certification lasts to know when it’s time to renew it.
(more…)
The science behind radioactive materials impacts human lives. In particular, carbon-14 is a prevalent radioactive isotope for medical purposes. They help with diagnostic and treatment solutions for patients. Let’s dive deeper to understand if the presence of carbon-14 can affect drug quality.
(more…)